Anemia is a condition characterized by a decrease in the number of red blood cells or hemoglobin levels, which poses significant health challenges, particularly among older adults. Although the causes and consequences of anemia are not fully understood, clinical evidence has indicated that anemia itself causes morbidity and may complicate other health conditions. The current treatment strategies include dietary supplements, blood transfusions, blood and bone marrow transplants, surgery, or erythropoiesis-stimulating agents. In the case of congenital anemias like Diamond-Blackfan anemia (DBA), Fanconi anemia (FA) and thalassemia (THAL) require hematopoietic progenitor transplants, preferably from HLA-identical siblings.
However, challenges such as the lack of compatible donors and associated risks underscore the need for innovative approaches to enhance patient outcomes and reduce healthcare costs. Accordingly, researchers from the University of Murcia (UMU) in collaboration with the IMIB and CIBERER have discovered a new molecular pathway that controls the formation of blood cells (or hematopoiesis), offering the potential for repurposing existing drugs to be used for new therapeutic purposes. Particularly, the repurposing of several tyrosine kinase (MAP3K20) inhibitors, that are approved by regulatory agencies FDA/EMA, for use in the treatment of congenital anemias.
They have identified the NLRP1 inflammasome as an important regulator of hematopoiesis and that its genetic inhibition reduces anemia in preclinical zebrafish models of Blackfan-Diamond anemia (DBA). The identification of the mechanism of NLRP1 inflammasome activation in hematopoietic stem and progenitor cells (HSPC) through the ZAKα/P38 MAPK pathway has allowed the use of tyrosine kinase inhibitors (TKIs), widely used in the clinic for other hematological diseases, demonstrating that these TKIs could be effective as a treatment against congenital anemia.
Importantly, the mechanism discovered is conserved between zebrafish and humans, but not in mice. This underscores the importance of utilizing alternative preclinical models, such as zebrafish, and proceeding with clinical trials in human subjects.
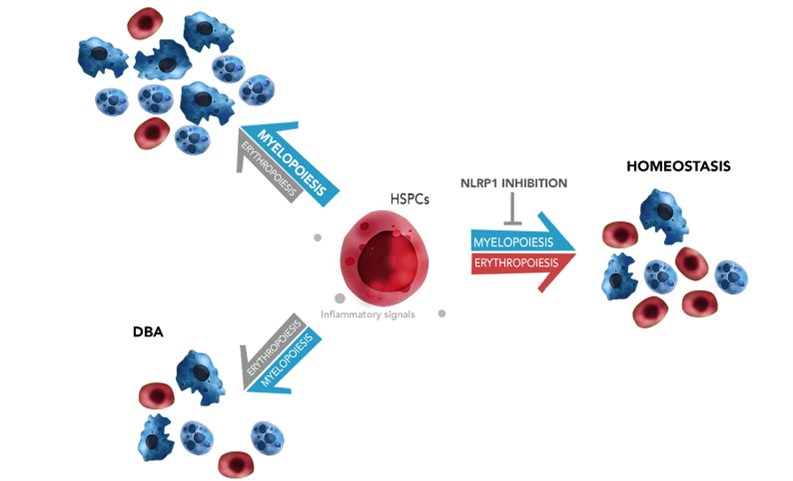
The efficacy of five TKIs in restoring defective erythropoiesis in DBA has been convincingly demonstrated through in vivo studies utilizing preclinical zebrafish models, as well as in vitro experiments involving HSPCs derived from DBA patients. Given the compelling outcomes of these investigations, there is a strong rationale for advancing to clinical trials to further evaluate the safety and efficacy of TKIs in treating DBA in human subjects.
Benefits:
- The identification of the inflammasome as a major regulator of hematopoiesis and the effect of its inhibition is of great clinical relevance for the treatment of congenital anemia.
- It provides a more accessible treatment option for patients with anemia and opens the possibility of extending the therapeutic strategy to other inflammation-related diseases.
- A variety of approved and marketed generic drugs could be used for other indications with effective results.
- The repurposed candidates are generic drugs, which means that it could be a faster and more cost-effective route to development.
The represented institution is looking for a collaboration that leads to commercial exploitation of the presented invention.
Institution: The University of Murcia (UMU), the Biomedical Research Institute of Murcia (IMIB), and the Center for Biomedical Research in Rare Diseases Network (CIBERER).
TRL: 3-4
Protection status: A European Patent Application has been submitted in 2023.
Financed: Collaboration in Proofs of Concept – Seneca Foundation
Contact: Elisa Sáenz / e.saenz@viromii.com

