Cardiovascular diseases are currently one of the leading causes of global mortality, and a primary risk factor associated with these diseases is the accumulation of low-density lipoprotein cholesterol (LDL). Despite the prevalent prescription of statin-based therapies for cholesterol reduction, a substantial proportion of patients—approximately 80%—fall short of achieving target levels due to poor treatment adherence. This non-adherence is frequently attributed to adverse effects associated with statin intolerance. The diagnosis of statin intolerance presents challenges due to the absence of established biomarkers or diagnostic tests, leading to inconclusive findings.
To address this problem, researchers from the Instituto de Investigación e Innovación Biomédica de Cádiz have developed a panel that combines miRNAs and clinical variables (like years of dyslipidemia, the presence of atherosclerotic cardiovascular disease, etc.) as biomarkers to design a method using these biomarkers for statin intolerance diagnosis in patients with cardiovascular diseases.
The identification of this intolerance enables avoiding the use of statins in these cases and opting for other alternatives, thereby improving the patient’s quality of life, and reducing healthcare costs associated with the inappropriate use of these drugs.
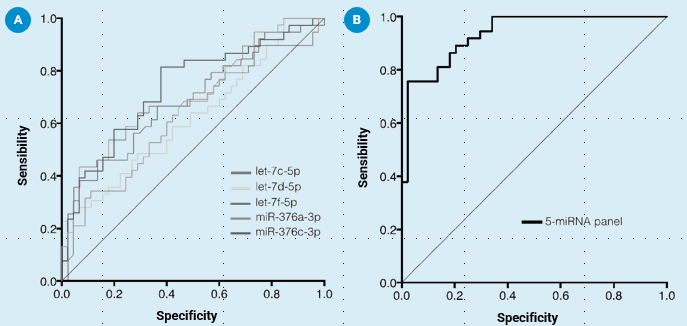
A study was conducted with 82 subjects, divided into two groups: statin intolerant (n=37) and statin tolerant (n=45), according to the criteria of the European Medicines Agency (EMA). In most of the panels studied, a diagnostic accuracy exceeding 80% was achieved, with the highest-performing one being a combination of panels, with an accuracy of 89.47%.
Furthermore, the proposed technology is protected by an international patent application PCT, which is expected to enter national phases (ES 2 953 481 A1) during 2024.
Benefits:
- miRNAS provide high stability and relevant information about epigenetic regulation and metabolic processes.
- High diagnostic capacity.
- Improvement of the patient’s quality of life.
- Savings in healthcare expenses by avoiding the administration of inadequate drugs.
The represented institution is looking for a collaboration that leads to commercial exploitation of the presented invention.
Institution: Instituto de Investigación e Innovación Biomédica de Cádiz (INiBICA)
TRL: 5-6
Protection status: Patent Application
Contact: Nuria Bas / nuria@viromii.com