Network medicine is the application of network science toward identifying, preventing, and treating diseases. It provides a better understanding of how diseases work and relate to each other. The Human Disease Network (HDN) represents a complex network where the nodes are the different diseases, and the edges are the relationships between them. The connections can be determined by genes, phenotypes, protein-protein interactions, drugs, and metabolic pathways, among others. However, de novo drug design is a highly time-consuming, costly, and challenging task. In contrast, drug repurposing (DR) offers a novel approach by identifying new potential uses for existing drugs, thereby reducing time and costs.
A group of researchers working in the Medical Data Analysis Laboratory (MEDAL) of the Polytechnic University of Madrid (UPM) has developed the GRENADA software.
This platform works as a large-scale disease network to better understand diseases and repurpose drugs. The main purpose is that it can be used by health professionals as a consultation tool, where they can find any kind of information, such as:
- List of existing/approved pathologies.
- Series of relationships of adverse effects.
- Interactions between drugs.
- How to relate a drug with the diseases.
Future ambitions:
- Carry out continuous integration and add new sources and data types.
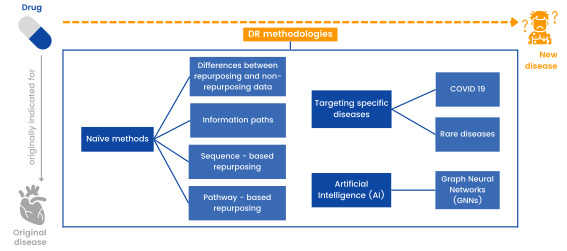
- Create a platform that brings repurposing methodologies together.
- Enhance AI models to obtain finer drug repurposing predictions.
- Perform experimental validation of the suggested repurposing hypotheses.
- Envision drug repurposing through the lens of personalized medicine.
The platform is being developed to bring all these drug repurposing methodologies together and subsequently, transfer this functional platform to a real product or service. The visualization of data and relationships is being generated, as well as the generation and explainability of the different hypotheses.
The ideal scenario is a collaboration with potential contacts from the pharmaceutical industry, drug regulatory agencies, patient associations, and policymakers to receive commercial feedback on their platform as part of the later potential technology transfer process.
Institution: Universidad Politécnica de Madrid
TRL: 2-3
Contact: Noelia Mas / noelia@viromii.com