Total laryngectomised/tracheostomised patients usually suffer from otorhinolaryngological pathologies associated with pulmonary disorders. They therefore require regular respiratory monitoring and evaluation using spirometric techniques. However, the spirometer adapters currently used for lung testing often consist of a tube and a necklace-like cord attached around the neck and are usually unable to establish an adequate connection between the spirometer nozzle and the patient’s laryngectomy/tracheostomy. This results in air leakage and unreliable results. These technologically outdated devices do not adapt to the patients’ anatomy and specific conditions.
Recently, researchers from the Instituto Murciano de Investigación Biosanitaria (IMIB) in Spain have developed a new alternative to the traditional spirometer adapters that improves test reliability. The invention consists of two parts that work together to connect laryngectomy/tracheostomy directly to the spirometer, creating a comfortable and easy-to-remove connection for patients.
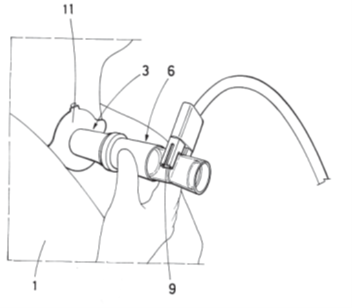
The device was clinically tested at the Hospital Universitario Reina Sofía of Murcia on 57 patients undergoing forced basal spirometry with successful results. Two 3D-printed models were developed, one reusable with replaceable filters and another with a build-in filter.
Further clinical trials are currently being coordinated by the Universidad Politécnica de Cartagena.
The technology is protected under Spanish utility model in 2021.
Benefits:
- The device can be used to perform a full range of respiratory tests.
- Its simple and flexible design can be easily adapted to patient’s physiology.
- Significantly improved results compared to those obtained from conventional methods due to the hermetic seal and air leak reduction.
- Reduced patient discomfort, allergic reactions, coughing reflex and nausea by filtering and minimizing the presence of microorganisms.
The represented institution is looking for a collaboration that leads to a commercial exploitation of the presented invention.
Institution: Instituto Murciano de Investigación Biosanitaria (IMIB)
TRL: 5-6
Protection status: Spanish Utility Model
Contacto: Noelia Mas / noelia@viromii.com

