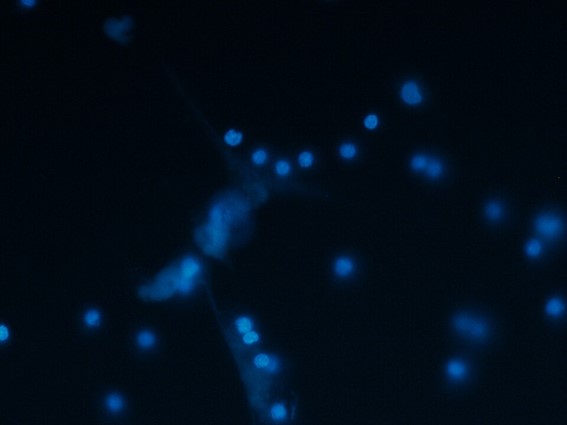
Neutrophil extracellular traps (NETs) are part of the immune system’s frontline defense, but their dysregulated formation (NETosis) contributes to various diseases. Excessive NETs are associated with thromboinflammatory conditions such as deep vein thrombosis, atherosclerosis, and sepsis, as well as autoimmune disorders and cancer metastasis. Within thrombi, NETs promote coagulation and hinder natural anticoagulants, leading to serious complications. Despite this, current treatments, such as DNase I or anti-inflammatory agents, are limited by incomplete NET clearance and off-target effects. Targeting NETosis at its source would be a more effective strategy, but no approved therapies currently address its core regulatory mechanisms.
Researchers from the Instituto Murciano de Investigación Biosanitaria (IMIB), the University of Murcia, and the Universidad Católica San Antonio de Murcia (UCAM) have identified a novel pharmacological strategy to inhibit the formation of neutrophil extracellular traps (NETs), which play a key role in thrombotic, inflammatory, and autoimmune diseases. The approach targets peptidylarginine deiminase 4 (PAD4), a critical enzyme involved in the process of NETosis. Using a structure-based drug repurposing strategy, the team identified gilteritinib, an FDA- and EMA-approved drug, as a potent PAD4 inhibitor. In vitro studies demonstrated that gilteritinib significantly reduces NET formation in human and murine neutrophils, limiting thromboinflammation. Unlike DNase-based therapies, which degrade NETs after they are formed, this strategy prevents NETosis at its origin, offering a promising new therapeutic avenue.
The technology has successfully demonstrated NET inhibition in in vitro studies. In vivo studies are ongoing to assess its therapeutic impact on thromboinflammation and mechanistic research is further validating its specificity.
Benefits:
- Root-cause targeting: Blocks PAD4 to prevent NET formation at its source.
- Repurposed drug: Uses gilteritinib, already approved by FDA and EMA, facilitating faster clinical use.
- Wide therapeutic scope: Applicable to diseases like thrombosis, autoimmunity, and cancer.
- Selective action: Preserves key immune functions by avoiding broad anti-inflammatory effects.
- Lower complication risk: Reduces NET-driven clotting and chronic inflammation.
- Limited treatment window: Potential for short-term use, minimizing long-term toxicity.
The represented institution is looking for a collaboration that leads to commercial exploitation of the presented invention.
Institution: Instituto Murciano de Investigación Biosanitaria (IMIB), the Universidad de Murcia (UM) and the Universidad Católica San Antonio de Murcia (UCAM).
TRL: 2
Protection status: Patent Application
Financing: IMIB’s own funds. Fundación Seneca, PoC.
Contacto: Nuria Bas / nuria@viromii.com