In the previous post, we explored the different preventive strategies pharma companies use to maintain their market exclusivity. At Viromii we feel this is a really interesting topic and in this post, we will continue the discussion by explaining the different innovation-linked strategies extracted from the article by Chie Hoon Song and Jeung-Whan Han “Patent cliff and strategic switch: exploring strategic design possibilities in the pharmaceutical industry.”
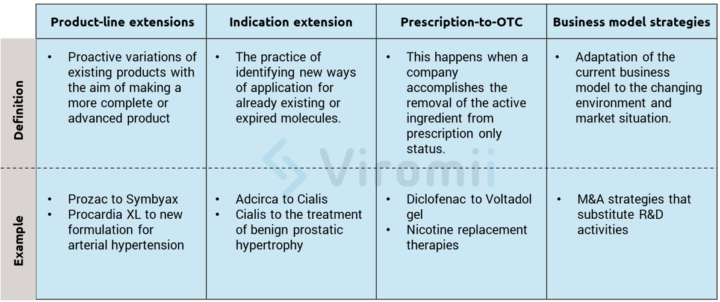
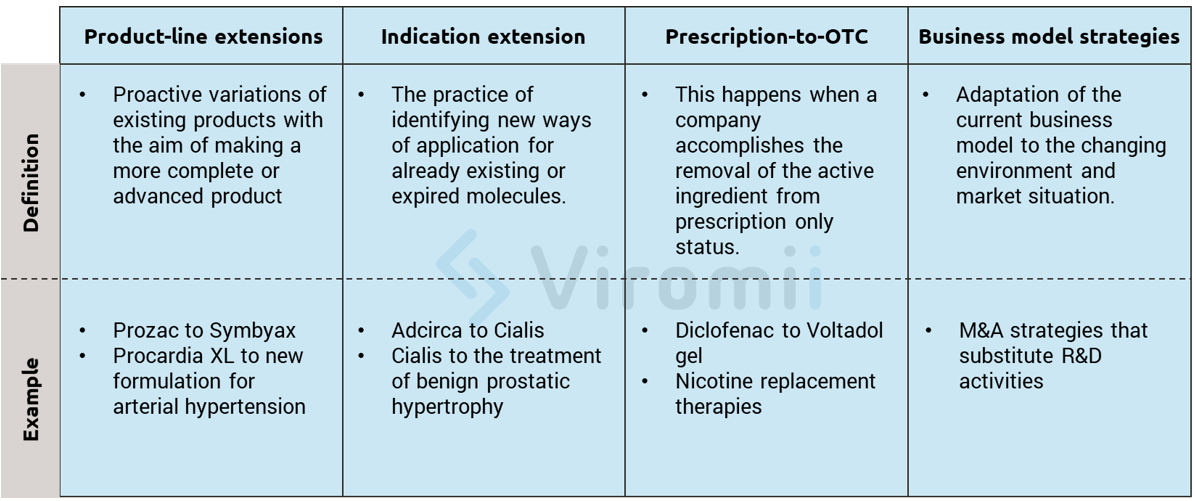
Innovation strategies focus on outpacing the competition by actively provoking shifts in the industry evolution and setting new market “rules” that competitors must constantly adapt to. Product-related examples of these strategies are product line extensions, approval of new indications, introduction of follow-on products, or prescription-to-OTC switch. These product-related examples all build upon current company’s assets and create new market opportunities. In the following lines, the previously mentioned examples will be further explained for a more comprehensive view.
Product-line extensions: These extensions can be better understood as proactive variations of existing products with the aim of making a more complete or advanced product. Examples include further purifying the product, lowering the costs of production through more modern production technologies, or creating superior formulations of already existing medicines. Here are some real-life examples of these strategies:
- Eli Lilly Prozac ® expired on 2001, so they obtained a patent for its combination with olanzapine and created Symbyax ® for refractory depression, moving the patent expiration year to 2017.
- Pfizer’s Procardia XL® is built upon a previous drug but with a new controlled-release formulation for the treatment of arterial hypertension.
Indication extension: This extension is a very “trendy” way of innovating in the drugs and molecules markets and refers to the practice of identifying new ways of application for already existing or expired molecules. This method really facilitates the regulatory process, which makes it of high interest for smaller companies. If the new clinical trials for the newly found indication appear to be promising, an extension of the drug approval may be requested. This way, the innovative company can gain three extra commercial exclusivity years, while at the same time extending their drug to new markets. There are thousands of examples of this process, including the one presented below:
- Eli Lilly’s Adcirca® was found to be useful with erectile dysfunction, so they created Cialis®. After some years of research they found a new dosing that was valid for benign prostatic hypertrophy and got that indication, granting them several extra years of commercial exclusivity.
Prescription-to-OTC switch*: This switch happens when a company accomplishes the removal of the active component from prescription only status. Therefore, by usually reducing the active component dose on the new OTC form, they open a new expansive market segment. The reasons behind these decisions vary, and may include less price negotiation, bigger patient base that can access the component, and, of course, the use of the brand loyalty from the previous prescription medicine to leverage on. There are risks and difficulties however. Obtaining OTC status usually requires very high proof of safety and high investment in marketing and advertising, which requires a big business adaptation since OTC products are very different from a prescription product. One important example of this switch can be found with the adaptation of drugs for muscular pain into topical gels.
* OTC: Over-the-counter drugs, drugs sold directly to the consumer, without the need of a prescription.
Business model strategies: These strategies involve many changes in the structure and governance of the way a company creates and captures value. In this case, adaptation is a must, and a company has to have their eyes wide open to be able to perceive changes in the market to quickly adapt to them. An example of this is the change in the paradigm that occurred when exploring and discovering NME (new molecular entities). As the prices and early regulatory processes increasingly became risky and costly, many companies began to adapt new business models based on licensing, ad-hoc commercialization, or acquisition of smaller firms that are more advanced in the regulatory process for their molecules. A simple example that we already discussed in our previous post is the case of the pharmaceutical company, Shire that decided to focus on rare diseases since it was a very niche field with big regulatory and exclusivity advantages.
We hope you enjoyed the read and will keep you updated with future posts. Follow us to know more!
Sources:
Song, C. and Han, J. (2019). Patent cliff and strategic switch: exploring strategic design possibilities in the pharmaceutical industry.